Gentleman,
Attached follows a schematic showing two situations;
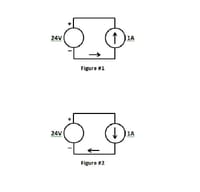
The figure #1 is showing a 1A current flowing in direction to the “+” pole of a voltage source, and due to that we may say that the voltage source (e.g. a lead-acid battery) is being charged.
On the contrary, the figure #2 shows a 1A current flowing in direction to the “-” pole of a voltage source.
Due that may we say that the voltage source (e.g. a lead-acid battery) is being discharged?
Thank you by your time.
G. L. Serodio
Attached follows a schematic showing two situations;
The figure #1 is showing a 1A current flowing in direction to the “+” pole of a voltage source, and due to that we may say that the voltage source (e.g. a lead-acid battery) is being charged.
On the contrary, the figure #2 shows a 1A current flowing in direction to the “-” pole of a voltage source.
Due that may we say that the voltage source (e.g. a lead-acid battery) is being discharged?
Thank you by your time.
G. L. Serodio