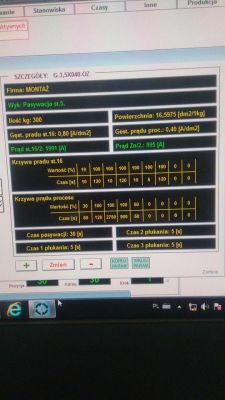
Hello.
Recently, I have been thinking a lot about the galvanization of metal things, filter baskets to be exact.
I would like to make a home-made galvanizing bath.
Namely, I'm interested in how to go about it.
The question is whether the bathtub can be made of ordinary sheet metal or plastic? What acid should be in the bathtub for the electrolysis to take place?
What transformer power (with what current) should I use in a 3x2x1m tub?
Does the voltage value matter?
The previous design of the machine was successful and thank you all for helping with the previous design.
I would like to ask for your help in this project.
Regards.
Recently, I have been thinking a lot about the galvanization of metal things, filter baskets to be exact.
I would like to make a home-made galvanizing bath.
Namely, I'm interested in how to go about it.
The question is whether the bathtub can be made of ordinary sheet metal or plastic? What acid should be in the bathtub for the electrolysis to take place?
What transformer power (with what current) should I use in a 3x2x1m tub?
Does the voltage value matter?
The previous design of the machine was successful and thank you all for helping with the previous design.
I would like to ask for your help in this project.
Regards.