FAQ
TL;DR: Polishing Cr-Co frames succeeds at 15.8 V/3.5 A [Elektroda, Bobo[PL], post #19943554][Elektroda, Bobo[PL], post #19945181]; "magnets cannot be heated" [Elektroda, Bobo[PL], post #19452539] DIY electrolytic polishers marry a modular 24 V-6 A PSU with PET-G/PLA 3-D parts and a stirrer for uniform gloss. Build cost stays under €120.
Why it matters: A tuned voltage-current pair speeds mirror-finish results while avoiding alloy pitting or magnet failure.
Quick Facts
• Working voltage: 15.8 V DC for Cr-Co alloy [Elektroda, Bobo[PL], post #19943554]
• Target current: 3.5 A for average denture frame [Elektroda, Bobo[PL], post #19945181]
• Electrolyte mix: ethylene glycol + H₂SO₄ + HCl (ELEKTROL) [Elektroda, Bobo[PL], post #19451278]
• 3-D print layer height: 0.2 mm using PLA/PET-G [Elektroda, Bobo[PL], post #19451278]
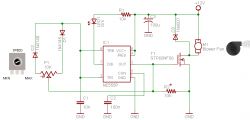
• Power source: 24 V 6 A modular SMPS inside Hammond aluminum case [Elektroda, Bobo[PL], post #19451278]
Which alloys polish well in the presented setup?
The ELEKTROL bath is formulated for Cr-Co-Mo dental alloys [Elektroda, Bobo[PL], post #19469372] Nickel alloys also work, but many labs avoid nickel because of allergy risk [Elektroda, Bobo[PL], post #19456939] Copper- or tin-rich alloys need a different bath—typically phosphoric acid, chromic anhydride and thiourea [Elektroda, Bobo[PL], post #19469609]
What voltage and current should I use?
Set the supply to 15.8 V and limit current to about 3.5 A for a single denture frame [Elektroda, Bobo[PL], post #19943554][Elektroda, Bobo[PL], post #19945181] Increase current only when polishing larger surface areas to keep current density around 0.2–0.4 A cm⁻², a value common in electro-brightening handbooks “Electropolishing Guide”.
How do I calculate safe current density?
- Measure the total wetted surface area (cm²). 2. Multiply by 0.3 A cm⁻² (mid-range). 3. Set the current limit to that value. Exceeding 0.6 A cm⁻² risks pitting and matte spots—an edge-case failure noted in plating literature “Electrochemical Surface Treatment”.
Are PLA or PET-G stirrer parts chemical-resistant enough?
PLA softens in hot acids, so print submerged parts in PET-G. The author sealed magnets in PET-G halves with epoxy, declaring “magnets are safe that way” [Elektroda, Bobo[PL], post #19454265] PET-G survives short exposure to diluted mineral acids up to 60 °C “PETG Chemical Resistance Chart”.
How is the magnetic stirrer built?
A PC-type 80 mm fan holds a PLA hub with three 10 × 3 mm magnets pressed and glued [Elektroda, Bobo[PL], post #19452539] The vessel stir bar is a PET-G cylinder enclosing three 4 × 4 mm magnets, halves glued with epoxy [Elektroda, Bobo[PL], post #19453841]
Could I just buy a Teflon stir bar instead?
Yes. Commercial PTFE-covered bars cost €2–€4 and resist nearly all lab acids [Elektroda, janek_wro, post #19457058] DIY bars are cheaper but risk leakage if the print has gaps.
What safety measures stop electrolyte spills from reaching live parts?
The top grille accepts a hood-filter pad that blocks splashes [Elektroda, Bobo[PL], post #19452539] Always place the jar in a secondary plastic tray and use a GFCI outlet; sulfuric acid can track along wiring, causing shorts.
How do I run a polishing cycle?
- Clip the workpiece as anode; insert the lead or copper cathode cylinder. 2. Fill the beaker with ELEKTROL, start the fan stirrer, set 15.8 V and 3.5 A. 3. Use the encoder to select time in 30-s steps; rinse and neutralise afterwards [Elektroda, Bobo[PL], post #19451278]
What happens if magnets overheat?
Neodymium magnets lose magnetisation above 80 °C; the builder warns “magnets cannot be heated” [Elektroda, Bobo[PL], post #19452539] Overheating stalls the stirrer and may fling the bar, contaminating the bath—an edge-case that ruins surface finish.
Is there a rule-of-thumb table for other metals?
Yes, but each alloy family needs unique acid ratios. Example: copper alloys polish at 6–9 V in phosphoric–chromic baths [Elektroda, Bobo[PL], post #19469609] Stainless steel often needs 9 A dm⁻² in a 50 % phosphoric–25 % sulfuric mix “Stainless Electropolish Spec”.
How much does the whole build cost?
Approximate parts list: SMPS €25, Hammond case €22, Arduino-based timer €12, fan and magnets €5, PLA/PET-G filament €8, misc. hardware €15. Total ≈ €87 plus lab glassware (component prices from EU retailers, 2023).













Comments
A stirrer with these 3 neodymium mates does the same? Is it for sale? If so, how did he sink the magnets and in what material? [Read more]
Nice made, but I have concerns about the air vent at the top of the case. I know that if you work carefully, you shouldn't spill anything, but there is a risk, and there is power inside. [Read more]
Alone. An element with magnets, printed with PLA filament, fitted to the fan "press-fit" and glued with poxipol. Then I heated the places for the magnets with a propane-butane burner and flooded them.... [Read more]
My colleague did not understand the question, or I asked incorrectly, so I will clarify it. I mean the stirrer in the vessel, as my colleague called it "jumping in the jar" made of 3 cylindrical 4x4 mm... [Read more]
forgive :) Printed two halves of a cylinder with a cylindrical recess inside for magnets. Both halves stuck together with poxipol. [Read more]
And that's what I meant :) I have been playing with 3D printing for some time, but I am wondering, firstly, whether such a printout will be tight (with you a magnet can corrode) and secondly, whether... [Read more]
Good question, but I must clarify that the stirrer element, in which the cylindrical magnets are closed, is made of PET-G. And I did so that first I pressed glue into the printed halves of the stirrer,... [Read more]
Where did the idea for such a useful device come from? In my opinion, it will be useful not only in dental prosthetics, but wherever you need to polish something irregularly shaped. Are you professionally... [Read more]
Thank you for your words of appreciation. I am a dental technician by profession. I used to make a few such devices, but back then I had no idea about 3D printing and they didn't have a stirrer. A... [Read more]
Why such combinations? Ready-made laboratory Teflon stirrers are available. They don't cost a fortune, chemically resistant Teflon. https://obrazki.elektroda.pl/3051506100_1622327451_thumb.jpg [Read more]
Cool equipment, tell what metals / alloys this electrolyte is suitable for, because on the manufacturer's website there is a note: And I would have a few details to polish, where the main alloy... [Read more]
Different metal alloys require different electrolytes and bath parameters. For copper, the electrolyte component is phosphoric acid, chromic anhydride and thiourea. Unfortunately, I have no experience... [Read more]
The current is shown on the display, what is the voltage? [Read more]
For chromocobalt around 15 ... 16V, so I took 15.8V. [Read more]
Thanks for the answer, and what current should not be exceeded? [Read more]
For dental technology purposes, 3.5A is ideal, but the current depends on the polished surface. You can experiment with the value of the current and the time. [Read more]
In all electrochemical processes of this type, the current density per unit area of the electrode is important. So the ampere alone is not enough ;) [Read more]